Abstract
Background: Exposure to drugs of abuse is frequently assessed using urine drug screening (UDS) immunoassays. Although fast and relatively inexpensive, UDS assays often cross-react with unrelated compounds, which can lead to false-positive results and impair patient care. The current process of identifying cross-reactivity relies largely on case reports, making it sporadic and inefficient, and rendering knowledge of cross-reactivity incomplete. Here, we present a systematic approach to discover cross-reactive substances using data from electronic health records (EHRs).
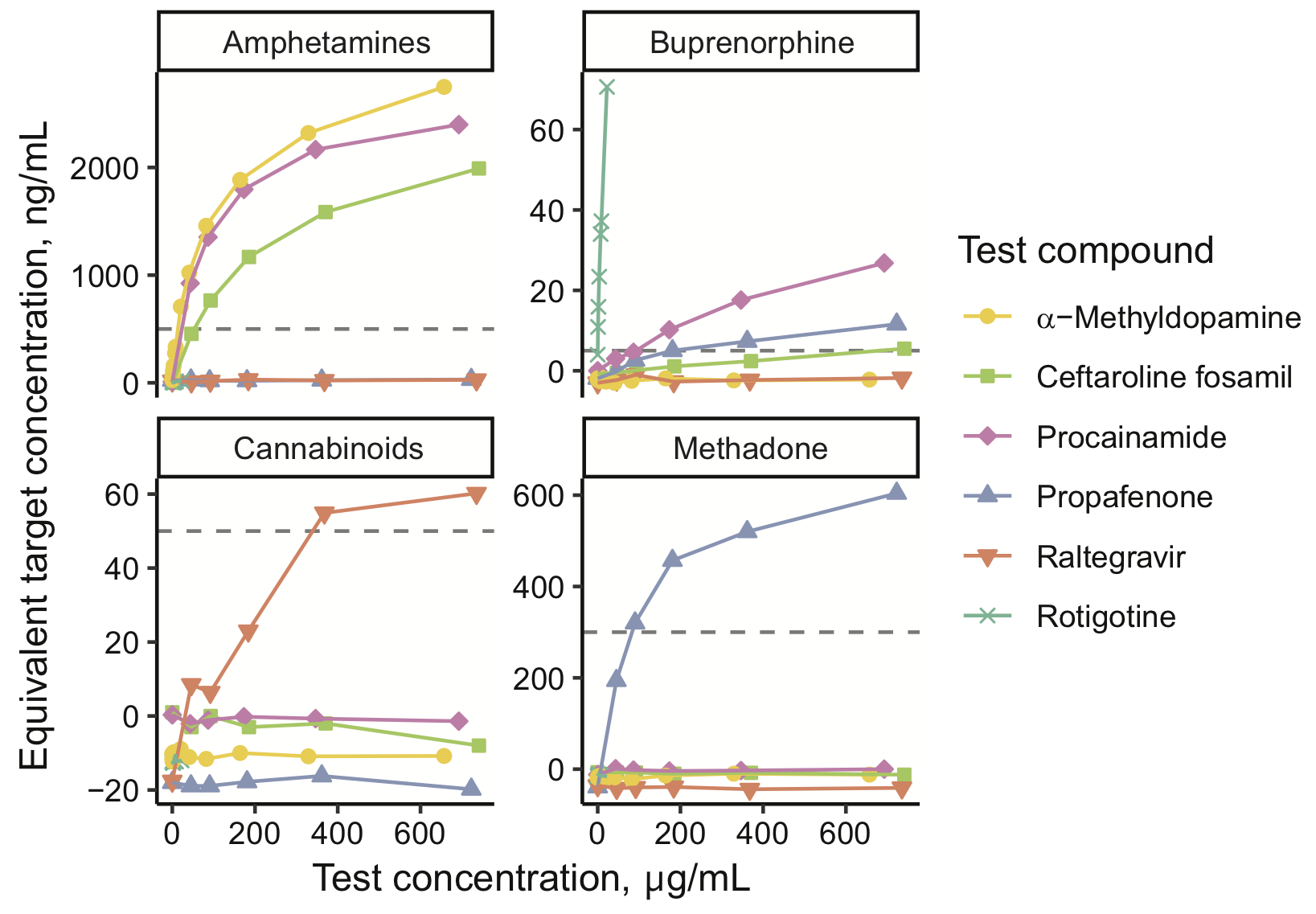
Methods: Using our institution’s EHR data, we assembled a data set of 698651 UDS results across 10 assays and linked each UDS result to the corresponding individual’s previous medication exposures. We hypothesized that exposure to a cross-reactive ingredient would increase the odds of a false-positive screen. For 2201 assay-ingredient pairs, we quantified potential cross-reactivity as an odds ratio from logistic regression. We then evaluated cross-reactivity experimentally by spiking the ingredient or its metabolite into drug-free urine and testing the spiked samples on each assay.
Results: Our approach recovered multiple known cross-reactivities. After accounting for concurrent exposures to multiple ingredients, we selected 18 compounds (13 parent drugs and 5 metabolites) to evaluate experimentally. We validated 12 of 13 tested assay-ingredient pairs expected to show cross-reactivity by our analysis, discovering previously unknown cross-reactivities affecting assays for amphetamines, buprenorphine, cannabinoids, and methadone.
Conclusions: Our findings can help laboratorians and providers interpret presumptive positive UDS results. Our data-driven approach can serve as a model for high-throughput discovery of substances that interfere with laboratory tests.
Where applicable, full text and supplement provided for fair use.